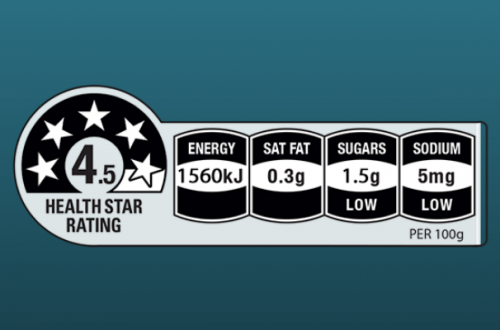
Since the health star rating was introduced in 2014 it has led to some confusion and controversy. This…
Read MoreBlog
News and advice
to help keep you healthy
Keep up to date with the latest on all things nutrition related by checking out our blogs that covers a range of interesting topics
Alcohol is one of those things that most people enjoy but knows isn’t great for their health. A…
Read MoreEndurance runnershave one thing in common… they all do large amounts of training to achieve their personal best!…
Read MoreFish is a high protein food that is often forgotten in our diets. Fish should ideally be consumed…
Read MoreNo doubt you have noticed an increase in plant-based milks on the supermarket shelf over the last few…
Read MoreDo you feel like no matter what you do to lose weight, your body is working against you?…
Read MoreThere is a general misconception that following a plant based diet will automatically result in deficiencies. A plant…
Read MoreMake a plan and get organised – organisation is the key to healthy eating. Think about what happens…
Read MoreWith the days getting shorter and colder, the easy thing to do is to head straight home from…
Read More